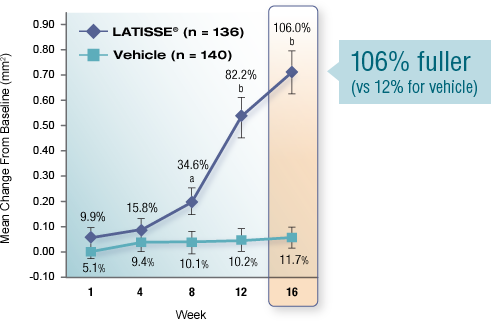
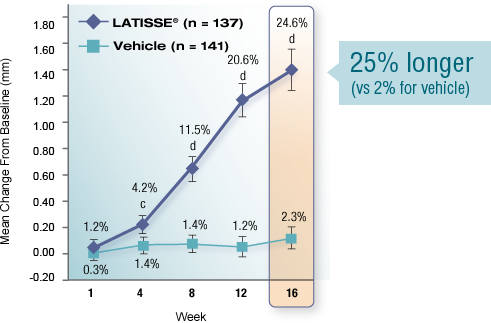
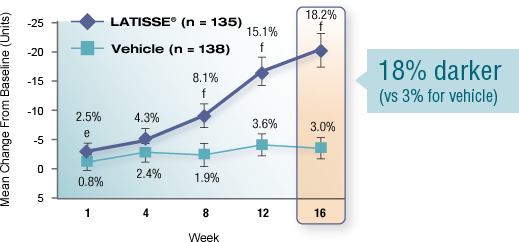
Real results in 16 weeks
Over-the-counter products condition eyelashes. In 2008, LATISSE® became the first and only prescription product FDA approved to grow eyelashes—fuller, longer, and darker—in patients with eyelash hypotrichosis.
91% of the 134 women on LATISSE® in the study were aged 35 or older. 61.9% had light eyes.
79.9% were Caucasian, 12.7% were Asian, 4.5% were Hispanic, and 3% were of a different ethnicity.
* Approximately 544,000 patients have purchased LATISSE®1,*
*Approximately 100,000 physicians have prescribed/dispensed LATISSE®2,*
* Approximately 4 million kits sold3,*
* 1 kit sold every 30 seconds, on average4,*,†
Important Safety Information
If you use/used prescription products for eye pressure problems, use LATISSE® under doctor care. LATISSE® may cause increased brown pigmentation of the colored part of the eye which is likely permanent. Eyelid skin darkening may occur and may be reversible. Only apply at the base of upper lashes. DO NOT APPLY to lower lid. Hair may grow on skin that LATISSE® frequently touches. If you have eye problems/surgery, consult your doctor about use of LATISSE®. Common side effects are itchy and red eyes. If discontinued, lashes gradually return to previous appearance.
You might be interested at
- Exosomes Infusion Therapy
- Sofwave™ Ultrasound Treatment
- Microdermabrasion Treatment
- Hydrogen Antioxidant Treatment
- Bela MD Treatment
- Cynosure Picosure Laser Treatment
- Cynosure Picosure Tattoo Remove Treatment
- Thermage CPT RF Treatment
- Endymed 3DEEP® RF Intensif Microneedling Treatment
- EndyMed 3DEEP® RF Fractional Skin Resurfacing
- Endymed 3DEEP® RF Skin Tightening
- Dermal Filler Injection
- BOTOX® Cosmetic Injection
- PRP Injection Treatment
- IV Booster Therapy
- Mesotherapy Treatment
- Belkyra Injection
- LATISSE®
- Skin Tag Removal
- VISIA® Complexion Analysis
- EndyMed 3DEEP® Body RF Contouring and Cellulite Treatment
